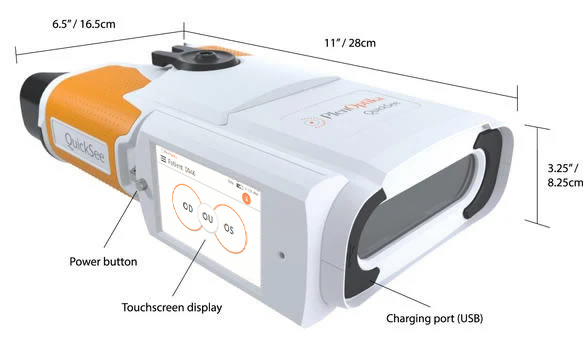
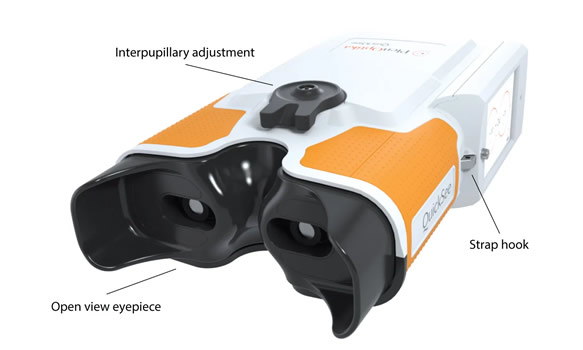
QuickSee by PlenOptika
Deliver better vision care and transform lives using the world’s most accurate handheld autorefractor.
Binocular and open view
Reduces patient accommodation for more reliable measurements
Wavefront aberrometry
Provides the most comprehensive method to measure ocular aberrations and refractive errors
Dynamic measurements
Produces results with high confidence
Affordable Autorefraction
As accurate as high-end clinical desktop autorefractors at a fraction of the price.
The patented PlenOptika Wavefront Refraction Engine™ performs continuous data analysis to precisely determine low-order refractive errors, making QuickSee as accurate as the high-end clinical desktop autorefractors and demonstrating excellent agreement with subjective refraction.
The technology inside QuickSee has been clinically-evaluated in five IRB-based studies and documented in seven peer-reviewed publications and conference abstracts. QuickSee has FDA Class I 510(k) exempt medical device registration.
Strong Agreement with Subjective Refraction
<= 0.25 D: 70-75% of patients
<= 0.5 D: 80-90% of patients (see publications)
Unmatched performance in the clinic and in the field
QuickSee’s combination of the open view binocular design, wavefront aberrometry, and dynamic measurements produce clinically accurate autorefraction measurements in a durable handheld format perfect for use in the clinic, optical shop, and mobile care – such as senior living centers or school settings.
Try QuickSee for 10 days and see how fast, accurate handheld autorefraction can help you grow your practice. Contact us about our pilot program.