NEW




NEW

Desktop power in a handheld autorefractor
Measurements in 10 seconds
Modern, intuitive operator and patient experience


Our handheld autorefractor’s combination of the open view design, wavefront aberrometry, and innovative measurement algorithms produces clinically accurate autorefraction measurements, in a durable handheld format suitable for use in clinics and in the field.
The patented PlenOptika Wavefront Refraction Engine™ precisely determines low-order refractive errors, making QuickSee Free as accurate as the high-end clinical desktop autorefractors and demonstrating excellent agreement with subjective refraction.
QuickSee Free’s technology has been clinically-evaluated in five IRB-based studies and documented in many peer-reviewed publications and conference abstracts. QuickSee Free has FDA Class I 510(k) exempt medical device registration.
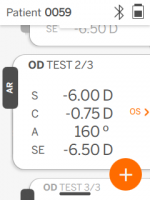
QuickSee Free’s UI is clean, intuitive, and robust
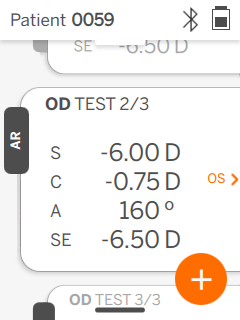
| Pupil size | 2 to 8 mm |
| Intended patient population | >= 3 years |
| Accommodation control | Open view, Fogging lens (optional) |
| Cycloplegia requirement | None |
| Dilation requirement | None |
| Illumination requirement | None |
| Acquisition time | 5, 10 seconds |
| Spherical range | -13D to +10D, increments of 0.01D, 0.125D, 0.25D |
| Cylindrical range | -8D to +8D, increments of 0.01D, 0.125D, 0.25D |
| Axial range | 0–180º, increments of 1º, 5º, 10º |
| Base technology | Wavefront aberrometry |
| Measurement modes (QuickSee Free Pro) | Simultaneous AR and K, AR only, and K only |
| Display properties | 2.4-inch LCD, capacitive touch screen, readable outdoors, true color (65,536 colors) |
| Charger properties | USB-C medical grade wall adapter, AC 100 to 240V, 50/60 Hz |
| Battery | 6 hours continuous use +/– 1 hour (10,000 mAh Li-ion); 3 hours charge time (5% - 75%); 5 hours charge time (0% - 100%); IEC 62133-2:2017 certified |
| Calibration | Factory calibrated; no field calibration needed |
| Measurement capacity | Measurement storage capacity: > 10,000 measurements |
| Regulatory classification (medical device) | Class I FDA (USA), Class IIa Product CE, MDR Compliant, Class IIa, UKCA, MDR 2002 Compliant |
| Laser safety | Class 1, IEC 60825-1:2014 certified |
| Weight | < 750 grams / 1.65 lbs |
| Dimensions | 30 cm (H) x 5.5 cm (W), 18 cm (L) |
Please note: Our Privacy policy and Terms of service were updated on September 30, 2025
Are you ready to learn how to use QuickSee Free / Free Pro effectively? Tell us about yourself and your circumstances and we will reach out to arrange a training session with you.